1. Soil Structure
What is soil structure?
Soil structure refers to how individual soil particles (sand, silt, clay) are arranged and bound together into aggregates called peds.
Types of soil structure
Soils are commonly grouped into these structural forms:
| Type | Description | Where it’s common |
|---|---|---|
| Granular | Small, rounded aggregates | Topsoil, rich in organic matter |
| Blocky | Cube-like peds | Subsoils |
| Prismatic/Columnar | Vertical columns | Clay-rich, arid soils |
| Platy | Thin horizontal plates | Compacted layers |
| Single-grained | Loose particles | Sandy soils |
| Massive | No structure | Heavy clays, compacted soils |
Why soil structure matters
Soil structure controls:
-
Water movement – infiltration & drainage
-
Aeration – oxygen supply to roots
-
Root penetration – ease of plant growth
-
Microbial activity – habitat for organisms
-
Resistance to erosion
Good structure = stable aggregates + good pore space
Poor structure = compaction, crusting, poor drainage
2. Clay Mineralogy
What is clay mineralogy?
Clay mineralogy is the study of the types of clay minerals in soil and how their crystal structure affects soil properties.
Clay minerals are layered silicate minerals with very small particle size and high surface area.
Main Types of Clay Minerals
1. Kaolinite (1:1 type)
-
One silica layer + one alumina layer
-
Low shrink–swell
-
Low nutrient-holding capacity (CEC)
-
Stable and non-sticky
-
Common in tropical, highly weathered soils
Soil behavior:
→ Easy to manage, but low fertility
2. Smectite / Montmorillonite (2:1 type)
-
Two silica layers + one alumina layer
-
High shrink–swell
-
Very high CEC
-
Absorbs lots of water
Soil behavior:
→ Very fertile but causes cracking, swelling, and foundation problems
3. Illite (2:1 type)
-
Similar to smectite but potassium holds layers together
-
Moderate swelling
-
Moderate CEC
Soil behavior:
→ Good fertility, moderate physical problems
4. Chlorite (2:1:1 type)
-
Extra hydroxide layer between sheets
-
Low swelling
-
Stable in cold or less-weathered soils
5. Oxides (Fe & Al oxides)
-
Not true clays but behave like them
-
Common in tropical soils
-
Give soils red/yellow colors
-
Strong phosphorus fixation
3. Relationship Between Soil Structure and Clay Mineralogy
Clay minerals strongly influence how soil structure forms.
| Clay type | Effect on structure |
|---|---|
| Kaolinite | Stable aggregates, good drainage |
| Smectite | Strong aggregation when dry, severe cracking and swelling |
| Illite | Moderate aggregation |
| Oxides | Very stable micro-aggregates |
4. Practical Importance
In agriculture
-
Determines fertility, irrigation needs, and tillage practices
-
Smectitic soils → fertile but hard to manage
-
Kaolinitic soils → easy to manage but need fertilization
In engineering
-
Swelling clays cause:
-
Cracked roads
-
Damaged foundations
-
Pipeline distortion
-
In environmental science
-
Clay minerals control:
-
Pollutant adsorption
-
Heavy metal retention
-
Carbon storage
-
5. Simple Summary
-
Soil structure = physical arrangement of soil particles.
-
Clay mineralogy = type of clay minerals present in soil.
-
Together, they determine:
-
Water behavior
-
Nutrient availability
-
Soil strength
-
Plant growth potential
Soil Structure
Soil structure describes the physical arrangement of soil particles (sand, silt, clay, and organic matter) into larger units called aggregates or peds. This arrangement influences key soil properties like porosity, water infiltration, aeration, root penetration, and erosion resistance. Good soil structure promotes healthy plant growth by allowing air and water to move freely, while poor structure can lead to compaction, poor drainage, or crusting.
Formation and Factors Influencing Soil Structure
Soil structure forms through natural processes such as:
- Biological activity: Roots, earthworms, and microorganisms bind particles with organic secretions.
- Physical processes: Wetting and drying cycles, freezing and thawing cause expansion and contraction, leading to aggregation.
- Chemical factors: Flocculation (clumping) due to ions like calcium, or dispersion due to sodium.
- Human impact: Tillage, compaction from machinery, or addition of organic amendments can enhance or degrade structure.
Key factors include soil texture (proportion of particle sizes), organic matter content, pH, and climate. For example, soils high in clay tend to form stronger aggregates but are prone to cracking when dry.
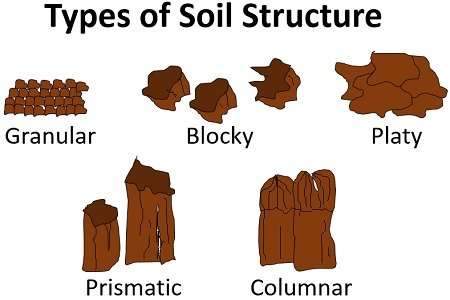
Types of Soil Structure
Soil structures are classified based on the shape, size, and stability of aggregates. Here's a summary:
| Type | Description | Common in | Characteristics |
|---|---|---|---|
| Granular | Rounded, porous aggregates (1-10 mm) resembling crumbs. | Topsoils with high organic matter, like in grasslands or forests. | Excellent aeration and drainage; ideal for root growth. |
| Blocky (Angular or Subangular) | Cube-like blocks with sharp or rounded edges (5-50 mm). | Subsoils in moderately weathered areas. | Good permeability; breaks easily when moist. |
| Prismatic or Columnar | Vertical columns or prisms (10-100 mm), often with flat tops in columnar form. | Arid or sodium-rich soils (e.g., in deserts). | Poor horizontal water movement; can indicate salinity issues. |
| Platy | Flat, plate-like layers (1-10 mm thick). | Compacted or forested soils with high silt. | Restricts vertical water flow and root penetration. |
| Massive | No distinct aggregates; cohesive mass. | Heavily compacted or clayey subsoils. | Poor aeration and drainage; difficult for roots. |
| Single-Grained | Loose, individual particles with no aggregation. | Sandy soils, like beaches or dunes. | High permeability but low water retention. |
These types can grade into each other, and soil profiles often show varying structures with depth.
To visualize these, here are some diagrams of common soil structure types:
Importance of Soil Structure
Strong structure enhances soil fertility by improving nutrient availability and microbial activity. Degraded structure, often from over-farming or erosion, leads to reduced crop yields and environmental issues like runoff. Management practices like cover cropping, reduced tillage, and adding compost can improve it.
Clay Mineralogy
Clay minerals are fine-grained (<2 μm) phyllosilicate (sheet-like) minerals that dominate the clay fraction of soils. They are secondary minerals formed from the weathering of primary silicates like feldspars and micas. Clay mineralogy studies their composition, structure, and properties, which profoundly affect soil behavior, including plasticity, shrinkage, swelling, and nutrient retention.
Basic Structure of Clay Minerals
Clay minerals consist of layered sheets of silica tetrahedra (SiO₄ units) and alumina octahedra (AlO₆ units). These layers stack in repeating units:
- Tetrahedral sheet (T): Silicon atoms surrounded by four oxygens, forming a hexagonal network.
- Octahedral sheet (O): Aluminum or magnesium atoms surrounded by six oxygens or hydroxyls. Layers are held together by weak van der Waals forces, hydrogen bonds, or interlayer cations, allowing for expansion or contraction.
Clay minerals are classified by layer ratio and interlayer features:
- 1:1 type: One tetrahedral sheet bonded to one octahedral sheet (e.g., kaolinite). No interlayer space; stable and non-swelling.
- 2:1 type: One octahedral sheet sandwiched between two tetrahedral sheets (e.g., montmorillonite). Expandable interlayer with water and cations; prone to swelling.
Isomorphous substitution (e.g., Al³⁺ replacing Si⁴⁺) creates a net negative charge, balanced by exchangeable cations (e.g., Ca²⁺, Na⁺), enabling cation exchange capacity (CEC)—a measure of nutrient-holding ability.
Common Types of Clay Minerals
Here's an overview of major clay minerals found in soils:
| Mineral Group | Structure Type | Key Composition | Properties | Occurrence |
|---|---|---|---|---|
| Kaolinite | 1:1 | Al₂Si₂O₅(OH)₄; mostly Al in octahedral sheet. | Low CEC (3-15 meq/100g), non-swelling, high stability. Used in ceramics. | Tropical, highly weathered soils; low fertility. |
| Montmorillonite (Smectite) | 2:1 (expandable) | (Na,Ca)₀.₃₃(Al,Mg)₂Si₄O₁₀(OH)₂·nH₂O; Mg/Fe substitution. | High CEC (80-150 meq/100g), swells with water, high plasticity. | Arid/semi-arid soils, volcanic ash; causes cracking in dry conditions. |
| Illite (Mica-like) | 2:1 (non-expandable) | K₀.₆₅Al₂(Si₃.₃₅Al₀.₆₅)O₁₀(OH)₂; potassium in interlayer. | Moderate CEC (10-40 meq/100g), fixed K⁺, less swelling. | Temperate soils from mica weathering; good K source. |
| Chlorite | 2:1 with interlayer brucite sheet | (Mg,Fe)₃(Si,Al)₄O₁₀(OH)₂·(Mg,Fe)₃(OH)₆; Mg/Fe rich. | Low to moderate CEC, non-swelling, green color. | Metamorphic or sedimentary soils; stable in acidic conditions. |
| Vermiculite | 2:1 (semi-expandable) | (Mg)₀.₃(Mg,Al,Fe)₃(Si,Al)₄O₁₀(OH)₂·4H₂O; hydrated Mg interlayer. | High CEC (100-150 meq/100g), expands when heated. | Weathered biotite soils; used in horticulture for aeration. |
Mixed-layer clays (e.g., interstratified illite-smectite) are also common, combining properties of multiple types.
For a better understanding, here are illustrations of the crystal structures of common clay minerals:
Properties and Soil Implications
- Cation Exchange Capacity (CEC): Clays adsorb and exchange nutrients like K⁺, NH₄⁺, aiding fertility. Smectites have the highest CEC.
- Swelling and Shrinkage: Expandable clays like montmorillonite absorb water, causing volume changes that can damage foundations or improve water retention.
- Plasticity and Cohesion: High clay content makes soil sticky when wet and hard when dry.
- Weathering and Formation: In humid climates, kaolinite dominates due to leaching; in drier areas, smectites prevail. Clay mineralogy influences soil classification (e.g., Vertisols with swelling clays) and management, such as lime application to flocculate dispersive clays.

